Reynoird Lab - Research
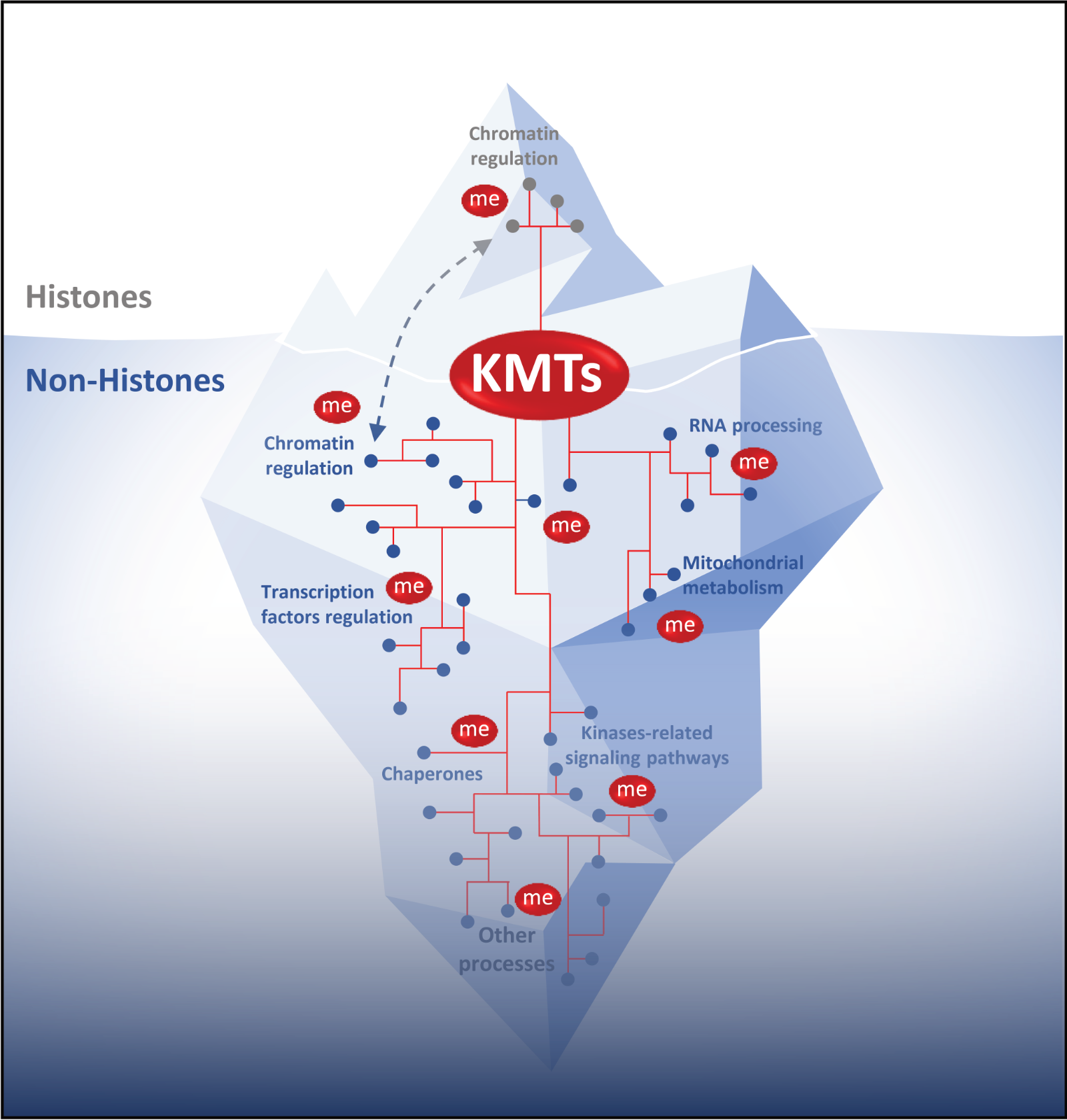
Lysine methylation signaling is a dynamic process thanks to the lysine methyltransferases (KMTs, catalyzing addition of methyl groups), lysine demethylases (KDMs, erasing such modifications) and specific methyl-binders (promoting appropriate responses). Lysine methylation of histone proteins are known to fundamentally regulate chromatin function, however there is a growing appreciation that a number of non-histone proteins undergo lysine methylation. Thus, it is likely that many proteins involved in cell homeostasis are methylated and that deregulation in protein methylation signaling may play a role in various diseases. Despite the fundamental role for this modification in biology, relatively few protein lysine methylation substrates have been identified and the function of lysine methylation signaling rarely characterized.
Our research aims to identify poorly described and potential KMTs involved in tumorigenesis and to characterize key and novel lysine methylation signaling in human cancer. The vast majority of lysine methyltransferases are still poorly characterized and for the most part their activity is unknown or not thoroughly analyzed. For example, we recently demonstrated that the lysine methyltransferases SMYD3 does not methylate histone H3 as previously claimed in literature, but has a clear oncogenic activity through the methylation of cytoplasmic protein such as the kinase MAP3K2.
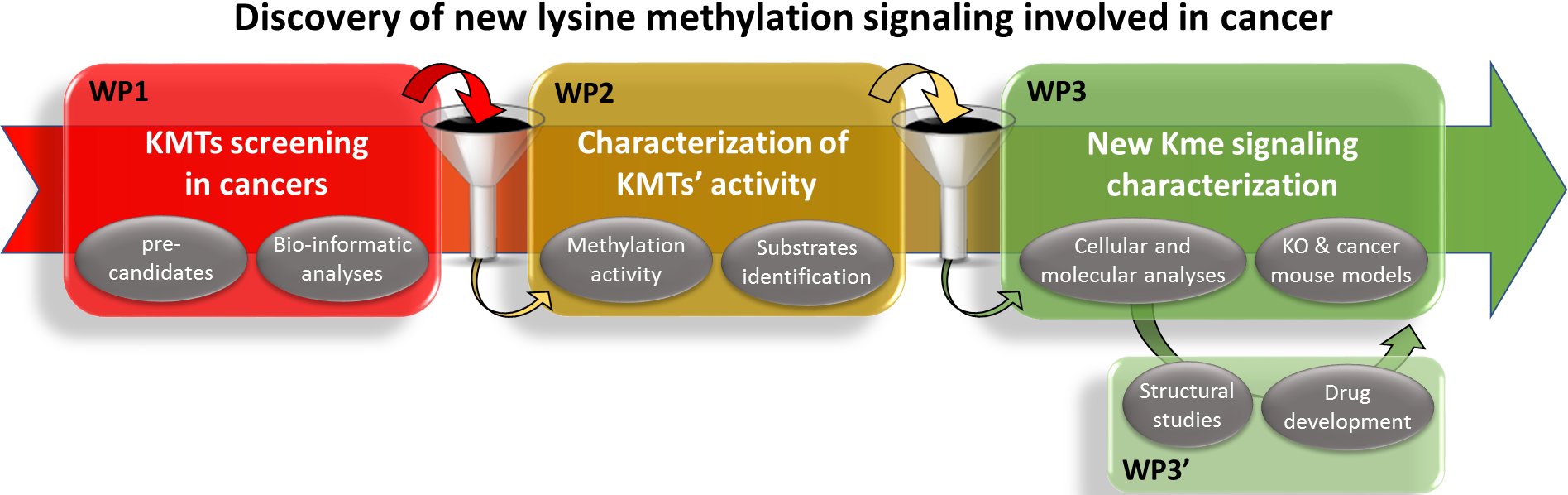
The long-term goal of our research is to demonstrate the under-appreciated importance of lysine methylation signaling in cell homeostasis and to offer new promising clinical targets for cancer treatment.
Our recent works focus on the discovery of new SMYD2 and SMYD3 signaling. We identified several new substrate candidates and we are now in the process of characterizing corresponding new lysine methylation signaling, involved in diverse biological functions including cell migration/invasion regulation and acquired resistance to chemotherapy and DNA damage.